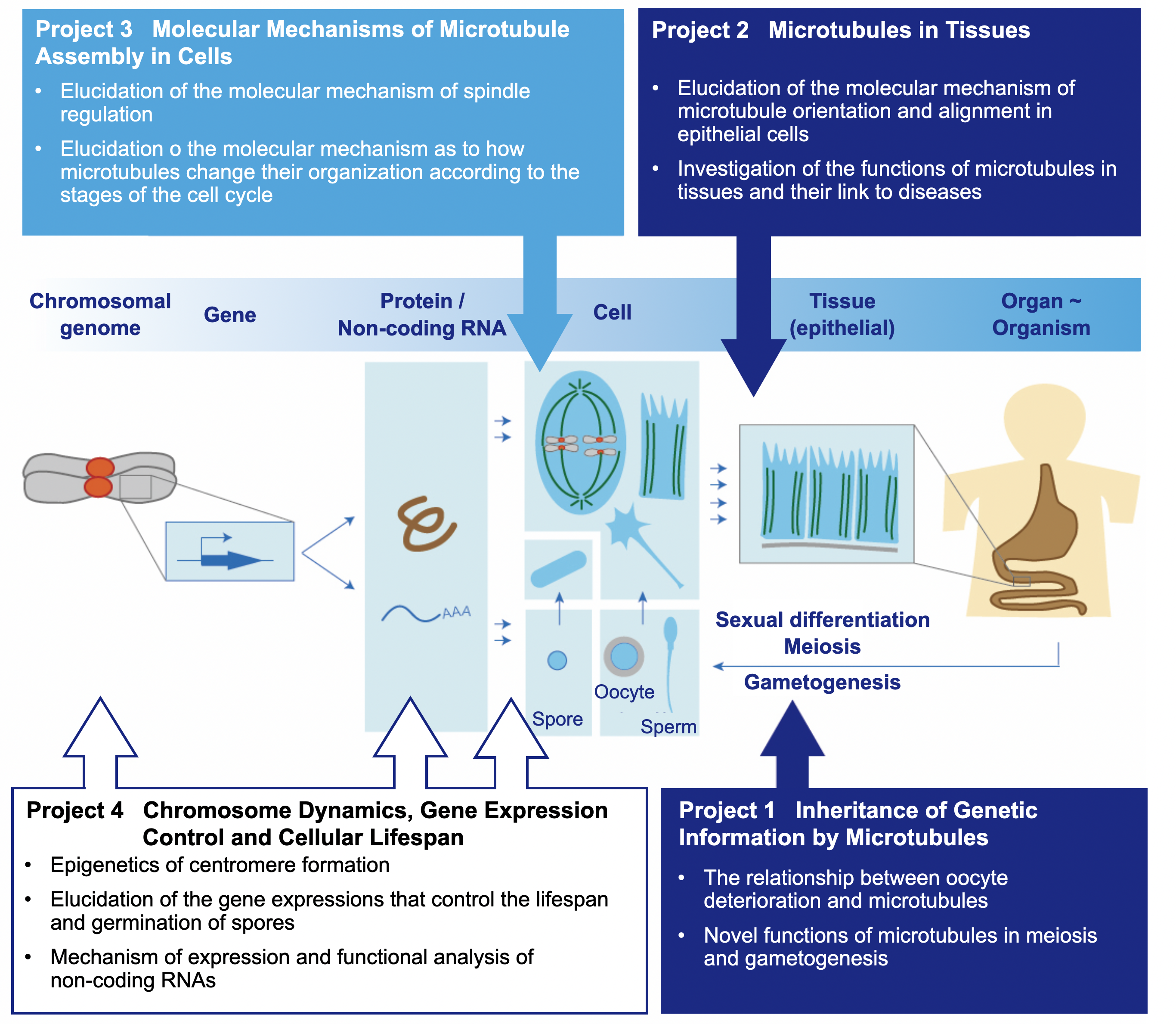
Our Research
Our body consists of numerous cells. We were born as a single cell called zygote, from which numerous somatic cells were produced through many rounds of divisions. Proliferated cells were then differentiated into various types of cells in various tissues and organs, thereby constituting a body comprising trillions of cells.
There are unanswered questions regarding behaviors of cell division and differentiation – how a new cell (daughter cell) is generated from an old cell (mother cell), and how tissues are properly assembled by a huge number of cells. We hope to gain molecular and cellular insights to understand the mysteries of life regarding cell division and body development. We particularly focus on cytoskeleton (microtubule, actin and intermediate filaments), which functions during cell division and tissue assembly.
What would happen to our body if cytoskeleton went wrong? Recent evidence demonstrates that some diseases are caused by abnormalities of cytoskeleton. Our laboratory studies the relationship between cytoskeleton and diseases to reveal mechanisms as to how diseases arise and develop. We dissect the entire story into several levels: molecules, genes, cells, tissues and individuals, and focus on each level as explained below in detail.
![]()
Eukaryotic cells undergo two styles of cell divisions: mitosis and meiosis. Mitosis is a division for proliferation of somatic cells, whereas meiosis is for production of gametes (sperms, oocytes and fungal spores). The cell division patterns switch from mitosis to meiosis depending upon environmental cues. Switching from mitotic cycles to meiosis is particularly called sexual differentiation. Defects in meiosis cause production of abnormal sperms and oocytes, to result in infertility, miscarriage and congenital chromosome abnormalities (trisomy such as Down syndrome). Investigation of mechanisms for meiosis has recently been attracting people from the viewpoint of development of reproductive medicine.
What is the difference between mitosis and meiosis in mechanisms? Meiosis has developed a number of unique systems so that haploid gametes can be faithfully generated. We would like to pinpoint genes that makes meiosis unique, through which we address the biological importance of such systems. We particularly focus on behaviours of chromosomes and the microtubule cytoskeleton, which are pivotal for progression of meiosis.
![]()
Most of previous researches on behaviours of cellular microtubules have been conducted using single cells such as yeast and cultured mammalian cells. This is mostly because observation and experiments using single cells are technically easier than those using tissue cells. For instance, yeast has advantages in easy genetic manipulation and in microscopy because of its transparency in colour.
On the other hand, our bodies are made of numerous cells that assemble tissues. The shape of individual cells in tissues are constrained by surrounding cells or the extracellular matrix, and each cell in tissues functions in such environments. We therefore consider that researches on microtubules using tissue cells are necessary to understand physiological functions of microtubules in our body.
We first investigate how microtubules are assembled and regulated in mouse epithelial tissues, as well as their functions. Epithelial tissues exist in most organs, including intestines, kidneys, lungs and livers. Epithelial tissue comprises a sheet of epithelial cells that maintain the spatial alignment along the vertical (apical to basal) axis. Inside epithelial cells, a number of microtubule filaments are aligned along the apicobasal axis to exhibit a characteristic organisation like a curtain. We focus on microtubules in epithelial cells to investigate the significance of the polarised microtubule alignment. In this project, we also shed light on possible linkages between microtubules and human diseases.
![]()
As mentioned above in Project 1 and Project 2, microtubules play essential roles in cell division and tissue morphogenesis. This means that assembly of microtubules is the trigger of those cellular processes. How are microtubules assembled in cells?
In non-dividing cells, microtubules are present in the cytoplasm. In contrast, the cytoplasmic array disappears upon entry into mitosis, which are then rebuilt into the spindle. Spindle microtubules capture kinetochores of duplicated chromosomes (sister chromatids) and pull them apart to separate them into two daughter cells. Chromosome segregation errors are closely associated with cancer, meaning that the spindle must be properly assembled, and microtubules need to be correctly controlled to ensure faithful segregation of chromosomes.
In this project, we aim to understand molecular mechanisms as to how microtubules dramatically change their organisation throughout the cell cycle. Our approach is based on genetics and cell biology using fission yeast and cultured human cells.
![]()
How do cells repeat to divide and maintain their life? It is not easy to address the question. Having discussed in Project 3, it is essential to duplicate all the chromosomes (genome) and to segregate them into two daughter cells by spindle microtubules. If kinetochores are abnormally assembled, microtubules cannot capture them, which results in segregation errors. There is a strict rule in number of kinetochores: only one kinetochore is allowed per chromosome, and it cannot be zero or more than one. This clearly insists that there is a regulatory system that allows assembly of one kinetochore per chromosome during cell division. The position of the kinetochore (called centromere) on a chromosome is unchanged and inherited over generations in an epigenetic manner.
A key factor for the mechanism to tell the position of kinetochores over generations is the histone H3 protein, which is a component of histone octamers, around which double strand DNA wraps to assemble nucleosomes in cells. Among several variants of histone H3, CENP-A is known as the centromere-specific variant, which exists only in the centromeric region. CENP-A is essential for kinetochore assembly: its binding to centromeric DNAs determines and inherits the site of kinetochore assembly.
The next question would be how CENP-A is recruited only to centromeres of chromosomes. Although the nucleotide sequence of the centromeric DNA contributes to acknowledging the site as a base for kinetochore assembly, it is not the sole factor. This is the reason why the phenomenon is called “epigenetic” inheritance, the position of CENP-A is inherited in a manner over genetic (sequence) information. The mechanism of epigenetic inheritance of CENP-A loading to centromeres over generations remains enigmatic. We would like to address the question in this project.
In relation to this, we also focus on non-coding RNAs (ncRNAs) that do not encode proteins. A lot of genes have been recently identified as ncRNAs, although their functions are poorly understood. We therefore aim to investigate ncRNA functions.
Going back to the question raised at the top of the section, we would like to find a clue to address the question from a different angle: how the lifespan of cells or individuals are determined is definitely one of the long-lasting question open to everyone in the world. Dividing cells will reach the limit after repeating rounds of divisions, which is called replicative lifespan. In contrast, quiescent (non-dividing dormant) cells are often able to maintain the viability for a long time, which is called chronological lifespan. When environment changes to break the dormancy, cells return to the cell division cycle. We hope to reveal how dormancy is broken in eukaryotic cells, and we have been focusing on yeast spores that remain dormant when nutritionally starved but germinate when nourished. Recently, we have shown that expression control of a histone H3 gene plays a pivotal role in efficient dormancy breaking in yeast spores.
Professor Masamitsu Sato
Assosiated Professor Mika Toya
