4.1 Kinetochore assembly founded by CENP-A
4.2 Analysis of fission yeast non-coding RNAs (ncRNAs)
4.3 Kis1 is required for CENP-A localization to centromeres, which then promotes stabilization of spindle microtubules
4.1 Kinetochore assembly founded by CENP-A
The kinetochore is a proteinous structure assembled on the centromere region of a chromosome. The kinetochore assembly is based on the centromere-specific histone H3 variant called CENP-A. Various studies have been carried out as to how CENP-A is localized to centromeres, but still there are many open questions. How is CENP-A recruited exclusively to centromeres? How is CENP-A inherited epigenetically if once recruited to centromeres over cell cycles? We would like to address these questions in this project (Fig. 4-1).
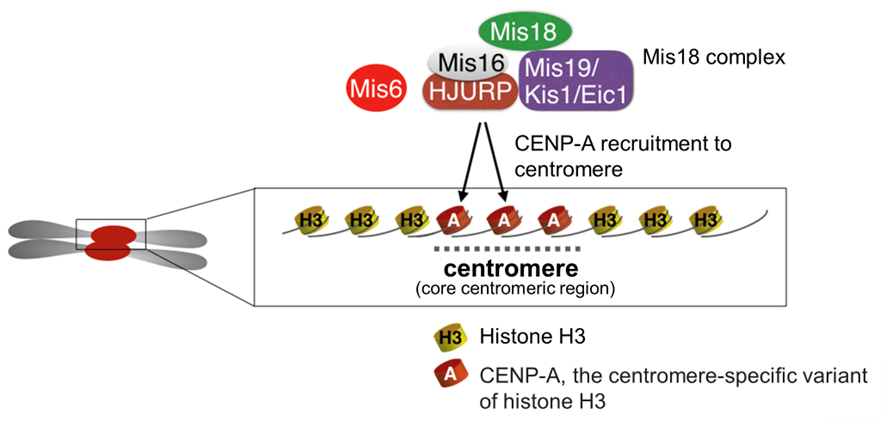
Figure 4-1 A centromere contains CENP-A nucleosomes, which serves as a platform for kinetochore assembly
Factors including the Mis18 complex recruit CENP-A, the centromere-specific variant of histone H3, to centromeres. The CENP-A nucleosomes serve as a platform for various kinetochore components.
We originally sought to identify novel regulators of spindle microtubules, and performed a genetic-visual screen using fission yeast. We isolated many mutants which displayed defects in organization of the spindle midzone, and one of those had mutations in a novel gene, which we named kis1. The gene was also identified by other groups studying kinetochore factors, and was named as mis19 or eic1. Kis1/Mis19/Eic1 is a component of the Mis18 complex, which plays an essential role in CENP-A localization. The kis1 mutant showed a significant reduction of CENP-A at centromeres.
In the kis1 conditional mutant, we found that most of the kinetochore components remained at centromeres, whereas CENP-A was displaced (Fig. 4-2). Mis6 (CENP-I) was also absent from centromeres in the kis1 mutant, and it has been known that Mis6 is also required for localization of CENP-A. Although it is known that CENP-A plays an essential role in kinetochore assembly, this result suggests that many other kinetochore components are able to remain at centromeres even when CENP-A is lost in the absence of Kis1 (the Mis18 complex).
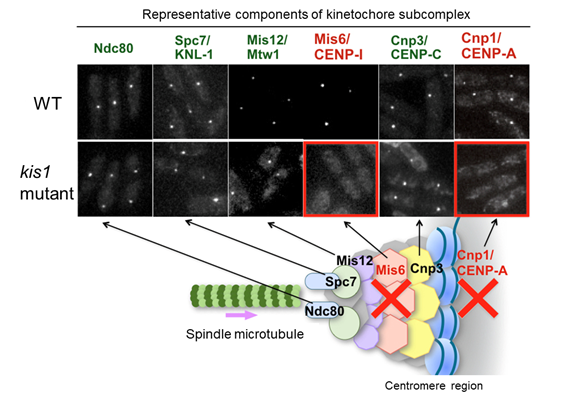
Figure 4-2 The kis1 mutant exhibits delocalization of CENP-A and Mis6, whereas other kinetochore components are unaffected
Localization of major kinetochore components was investigated in wild type (WT) and kis1 temperature-sensitive mutant cells. When visualized with fluorescent proteins, these components were normally observed as a punctate localization under the fluorescence microscope. In kis1 mutant cells, Mis6 and CENP-A (Cnp1) no longer localized to centromeres. In contrast, the localization of other kinetochore proteins (Ndc80, Spc7, Mis12, and Cnp3) was unaffected under the conditions.
In the kis1 mutants, most kinetochore components remained present without CENP-A for a while. Ndc80 and Spc7, representative kinetochore factors required for attachment to microtubules, remained normally at kinetochores in the absence of CENP-A in the kis1 mutant. Nonetheless, our analysis revealed that spindle microtubules often failed to attach the kinetochores without CENP-A. This finding seems slightly inconsistent with previous knowledge. We propose that not only well-known factors for microtubule attachment (such as Ndc80), but also CENP-A promotes attachment of microtubules to kinetochore.
At the beginning of the genetic screen, we isolated the kis1 mutant as a mutant which has a defect in spindle formation. Taking all the results into consideration, we now propose a mechanism in which CENP-A stabilizes spindle microtubules, through establishment of kinetochore-microtubule attachment (Fig. 4-3). Those results were published in PLoS ONE, and we are now investigating why kinetochore-microtubule attachment is defective in the absence of CENP-A, although Ndc80 and Spc7 remains normal. We also expand the study to address the open questions regarding CENP-A localization as listed at the top of the chapter.
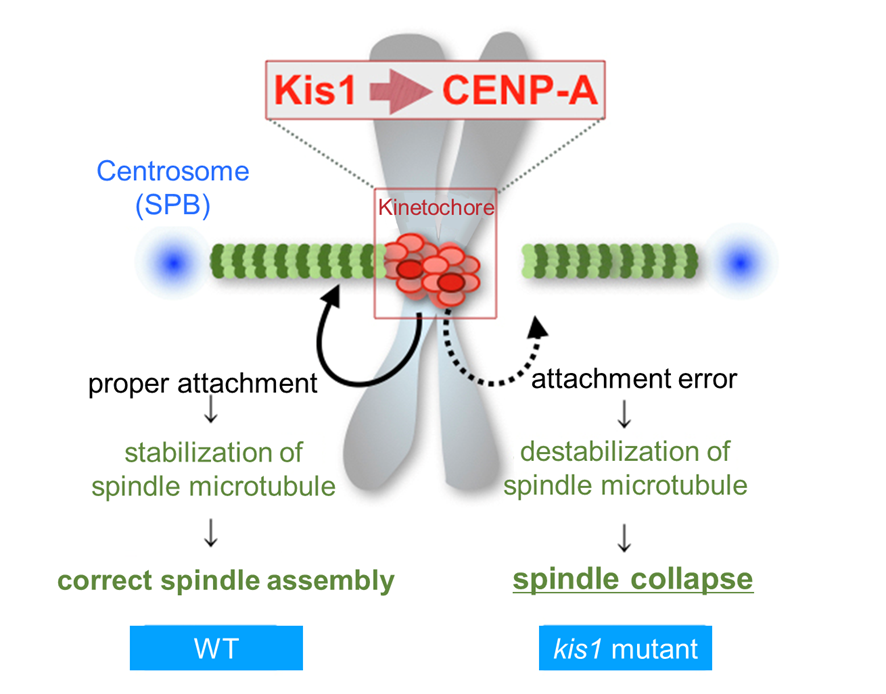
Figure 4-3 Kis1 is required for CENP-A localization to centromeres, which then promotes stabilization of spindle microtubules
In wild-type (WT) cells (left), the Mis18 complex containing Kis1 enables assembly of CENP-A nucleosomes at centromeres, which in turn promotes attachment of microtubules to kinetochores. When the attachment is properly made, the microtubules are stabilized. Conversely in the kis1 mutant (right), the kinetochores lack CENP-A, and the attachment is defective. Microtubules that failed to attach kinetochores are not sufficiently stabilized. This could be a major reason why the spindle is fragile in the kis1 mutant.
Related Publication:
The Kinetochore Protein Kis1/Eic1/Mis19 Ensures the Integrity of Mitotic Spindles through Maintenance of Kinetochore Factors Mis6/CENP-I and CENP-A
Hayato Hirai, Kunio Arai, Ryo Kariyazono, Masayuki Yamamoto and Masamitsu Sato
PLoS ONE (2014) 9(11):e111905. DOI:10.1371/journal.pone.0111905
Among the genes located on chromosomes in eukaryotic cells, there are a great number of non-coding RNA (ncRNA) genes that do not encode any proteins. Functions of ncRNAs have been largely unknown, and our study aims to elucidate them. We have introduced bioinformatic methods and launched our research aiming for comprehensive analyses of ncRNA functions.
