Eukaryotic cells do not always proliferate. Although they can actively proliferate depending on their surrounding environment, they terminate visible activities under some circumstances with unfavorable environments, and cells become “dormant”. Dormancy never means cell death. Cells indeed remain viable over a prolonged period of dormancy. Afterwards when the surrounding environment becomes suitable for growth, cells wake up and start proliferation. For example, plant seeds are dormant, and they germinate depending on the changes in their surrounding environment. In the case of animal cells, dormant cells in tissues may wake up in reaction to some unknown cues, and the dormancy breaking is considered as a trigger of carcinogenesis.
This means that switching proliferation and dormancy is a strategy for cells to survive even though the surrounding environment changes toward any direction. However, mechanisms by what molecules (genes) select their fate remain largely unknown.
We have conducted experiments (wet analysis) based on conventional genetics using the fission yeast Schizosaccharomyces pombe along with newly introduced experimental methods of advanced measurements. In addition, bioinformatics analysis (dry analysis) was carried out on the obtained data. Through the wet and dry analyses, we have successfully proposed a model on the molecular mechanism by which a cell wakes up from dormancy (Tsuyuzaki et al., Nature communications, 2020). We plan to further expand our experiments on genetics and molecular cell biology (wet analysis) in order to validate whether the model derived from the information analysis is accurate or not.
By combining traditional/advanced experiments (wet analysis) and information science (dry analysis) that includes statistics and artificial intelligence (AI), we hope to elucidate the mystery hidden behind cell fate determination.
4.3.1 Cellular dormancy and awakening
Eukaryotic cells repeat proliferation through cell division. However, when the surrounding environment of the cells changes, they exit the cell cycle and enter a state of dormancy, a state in which they stop dividing. In fact, eukaryotic cells that make up fungi, plants, and animals do not always proliferate, and dormant cells are often observed. Among such dormant cells, some are known to “wake up” and enter a proliferation state following environmental changes.
For example, plant seeds are a typical example of dormant cells. By being dormant as seeds, they can survive for a prolonged period of time even under environments unfavorable for growth (such as low-temperature or dry conditions). Then, when environmental conditions become favorable for cell proliferation, seeds wake up (germinate), and form a plant body. Moreover, many of the cells that structure our body are dormant as well. Part of these dormant cells waking up due to some unknown reasons is considered to be one of the triggers of carcinogenesis.
What kind of molecular mechanisms are involved in this cellular awakening? If the mechanism of cellular awakening can be elucidated, it is highly intriguing as a study of fundamental science, as well as agricultural and medical applications may be realized. For example, if unsuitable cellular awakening can be suppressed, it may potentially be applicable as a method of cancer treatment. To study cellular dormancy and awakening, we use fission yeast as the model organism in our research. With fission yeast, experiments can be conducted more easily compared to animals and plants, and cellular dormancy and awakening can be easily controlled.
When nutrients are available in the surrounding environment, fission yeast proliferate by repeating mitosis (Fig. 4-4). On the other hand, under nutrient deficient conditions, they halt proliferation and undergo meiosis to produce spores, which are gametes. In spores, intracellular metabolism is suppressed, and dormancy allows them to survive even under disadvantageous environments. However, when nutrients are supplied, they wake up and initiate a proliferation state through mitosis. This phenomenon is called spore germination.
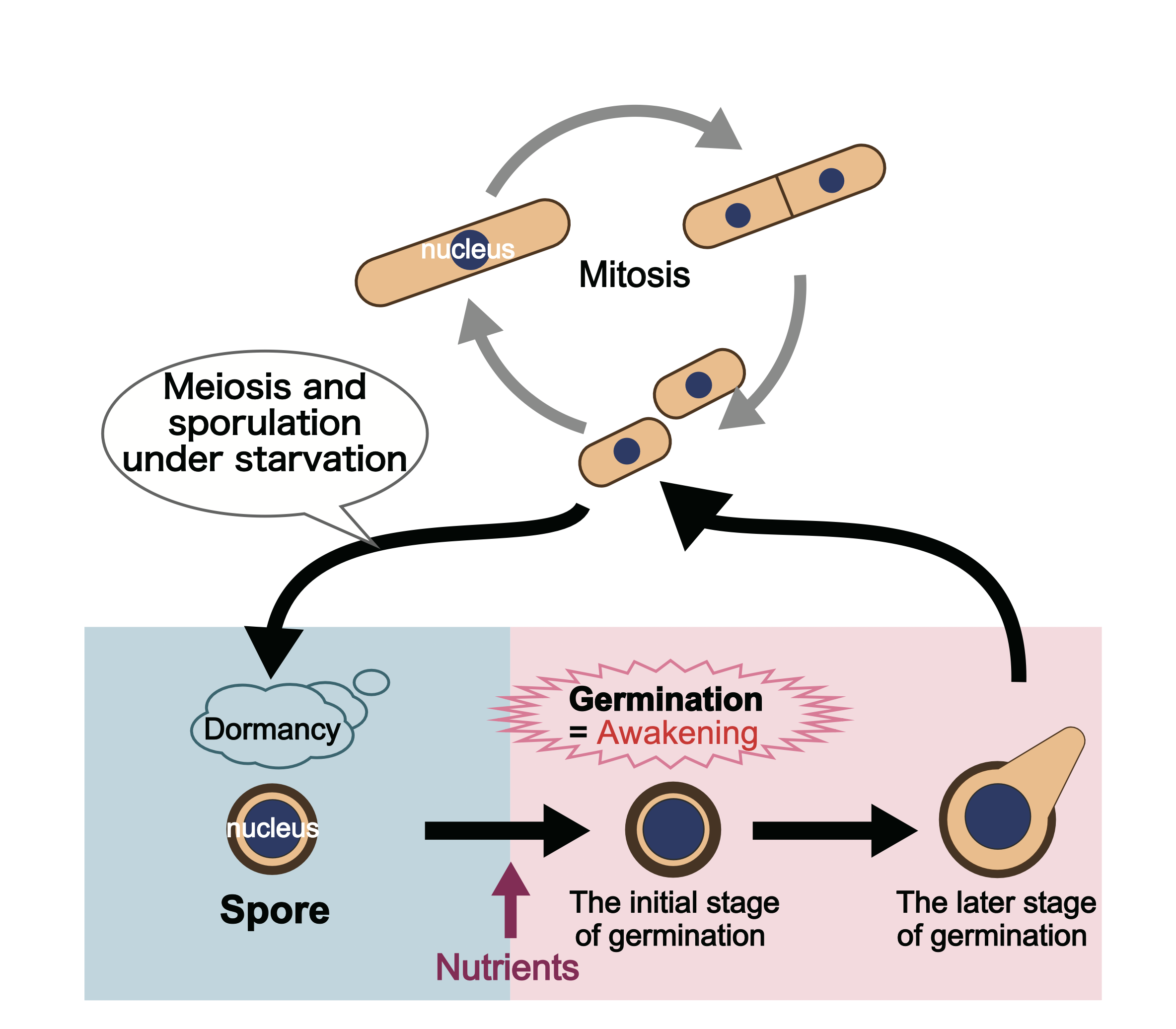
Figure 4-4 Cellular dormancy and awakening of fission yeast When nutrients are available in surrounding environments, yeast proliferate by mitosis. However, when nutrients are depleted, they halt proliferation and produce spores (gametes) through meiosis. Although spores are dormant cells that can withstand an environment disadvantageous for survival, when exposed to a nutrient-rich environment, they wake up (germinate) and enter a process of proliferation by mitosis.
In order to elucidate the molecular mechanism of cellular awakening, we considered investigating which genes are expressed to what extent in a cell, and thus, investigating the amount of mRNA for each gene will be the breakthrough. In particular, we assumed genes that increase (or decrease) their expression during the initial stage of germination are the genes that play a significant role in cellular awakening.
Data that lists how much each gene is expressed within a cell is called gene expression profile. In order to create gene expression profiles, RNA-seq (RNA sequencing), which utilizes next-generation sequencers to decode large quantities of mRNA sequences, has become common in recent years. Hence, it should be possible to generate a gene expression profile of germination by preparing a great number of spores at the initial stage of germination, collecting mRNA from the group, and conducting RNA-seq.
However, there was a problem. Conventional RNA-seq experiments had technical limitations, in which it required a great amount of mRNA to be prepared from a large number of yeast cells. Therefore, even if it is required to collect a large number of spores that are at the early stages of germination, this could not be achieved because the timing of germination varies among individual cells, and germination cannot be simultaneously induced in a population of spores.
This problem was solved by developing and introducing a method called single-cell RNA-seq (scRNA-seq) to fission yeast research. With the scRNA-seq method that has been developing in recent years, intracellular mRNA content can be investigated based on only a slight amount of mRNA extracted from a single cell. As this is possible, the need of preparing a large number of spores, as well as the need of synchronizing their timing of germination are not required anymore. At the time when we launched this research, there were no reported cases of conducting scRNA-seq using fission yeast, so we started from developing the technique itself. Our paper (Tsuyuzaki et al., Nature communications, 2020) is the first published study of scRNA-seq performed on spores.
4.3.3 Analysis using bioinformatics
We selected spores one by another, extracted mRNA individually from each of them, and created scRNA-seq profiles that listed the mRNA sequence and its abundance. A total of 64 spores were selected one by one, and 64 scRNA-seq profiles were generated. For fission yeast cells, there exists approximately 7,000 genetic information (“7,000 genes” for convenience) that are registered on the database. We were able to obtain big data of sequence information for 64 cells × 7,000 genes showing how much of each of those genes is expressed during germination.
To recall, we wanted to focus on the cells during the initial stage of the process of germination, and examine what genes exhibit changes in their expression (→ section 4.2.1, 4.2.2). Now, among the 64 spores selected under a microscope, which cells are at the very initial stage of cellular awakening? When selecting cells under a microscope, there are no distinct visual differences between any spores. Due to this reason, we were not able to judge at the point which cells correspond to the initial stage of germination. Thus, we made use of the techniques of bioinformatics to distinguish which cells among the selected 64 spores are at the initial stage of germination and which of them are at the middle or late stage. In other words, the idea is that by conducting AI analysis, the “degree of cellular awakening from dormancy” that cannot be judged by a cell’s physical appearance, as well as the information of their gene expression profile can be approximated according to the calculations (Fig. 4-5).
By comparing the 64 expression profiles based on calculations and lining them up in order with regards to similarity, we have successfully estimated a virtual timeline. This virtual timeline speculates the flow of change that informs that the 64 cells would have changed in this order with respect to time (Tsuyuzaki et al., Nature communications, 2020; Tsuyuzaki et al., Current Genetics, 2021).
As the cells at the initial stage of germination could be identified in this way, we were able to decide upon some genes whose expression level fluctuated during the initial stage of germination. Among them, we found that hht1 was included, which is one of the genes coding histone H3.
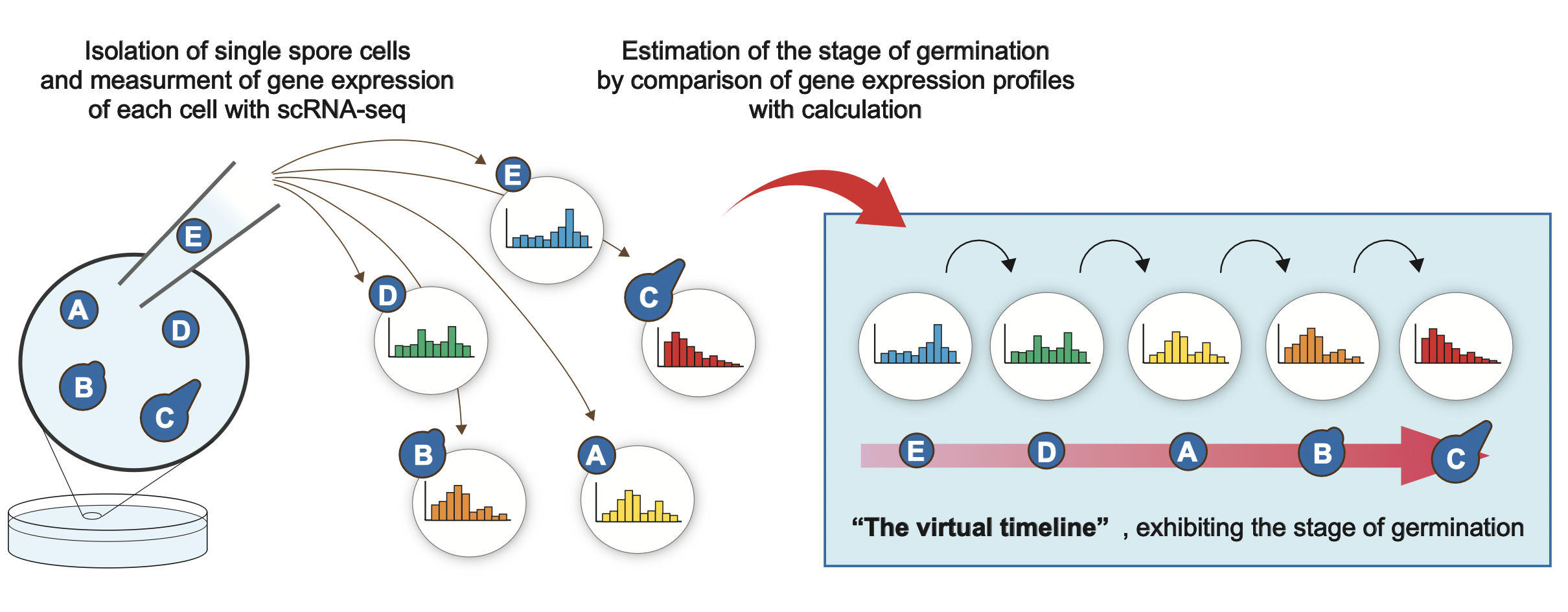
Figure 4-5 Estimation of a virtual timeline by using bioinformatics
Which stage of cellular awakening the spores are at (initial or middle stage) cannot be visually distinguished even when cellular morphologies are observed under a microscope. Thus, by measuring the gene expression level based on the scRNA-seq from each cell and by conducting bioinformatics analysis, we have successfully estimated a virtual timeline that indicates which stage within the time series of cellular awakening each cell is at. By this, detecting which cells among the 64 selected spores are at the initial stage of cellular awakening became possible.
4.3.4 Histone H3 that have emerged as a gene that promotes cellular wake up
Histone is a DNA-binding protein, and plays a role in regulating DNA expression. Eight histone proteins associate to form a histone octamer, and the structure in which DNA is coiled around the octamer is called a nucleosome (Fig. 4-6). For the entire chromosomal regions, DNA and histone octamer create nucleosome structures, but it is considered gene expression level changes by controlling the bonding state of histone octamers for chromosomes (chromatin).
Histone H3 is one of the building blocks of histone octamer. In fission yeast, there exists three histone H3 genes (hht1, hht2, hht3) on the genome. As the amino acid sequence of the protein these three types of genes encode are identical, there were no findings whether these three genes simply exist in a duplicated way, or functionally used differently. We discovered that among the three histone H3 genes, the expression level of hht1 decreases during the initial stage of germination, and increases again during the middle to late stage of germination. In contrast, the expression levels of hht2 and hht3 do not change much throughout the germination process.
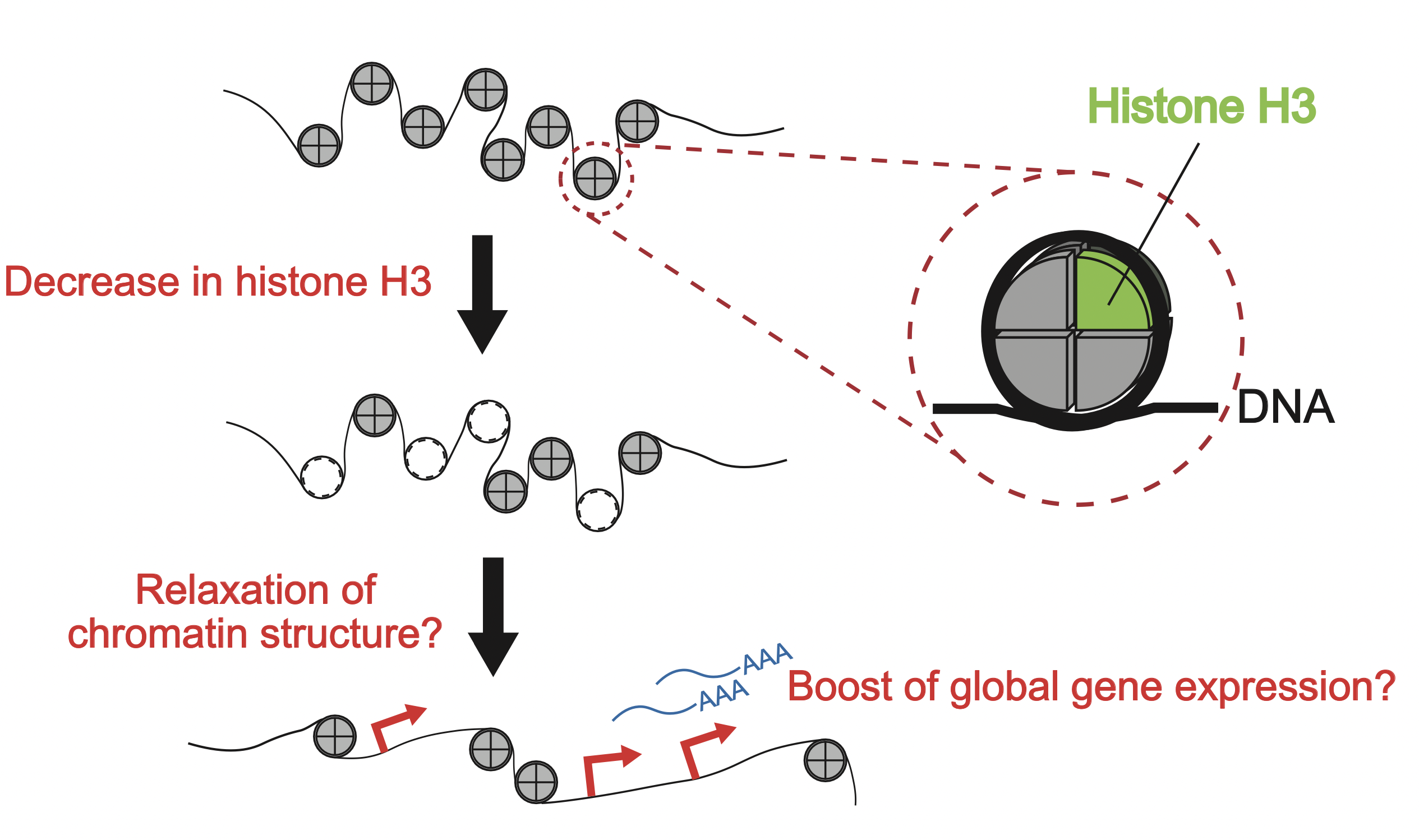
Figure 4-6 A possibility of gene expression regulation by changes in the state of nucleosomes
Histone H3 is one of the building blocks of histone octamer. We consider that as histone H3 decreases with cellular awakening, chromosomal structures loosen and gene expression is promoted.
4.3.5 Whether the expression of histone H3 should decrease or increase
Next, we deleted the hht1 gene (knock-out) and created spores that do not express hht1 at all. Observation of these spores revealed that while there were no abnormalities with the process of proliferation by mitosis, spore germination was remarkably delayed. Hence, among the three histone H3 genes, it can be suggested hht1 is an important gene for a cell to wake up. It may seem the function of these three histone H3 genes are the same since they encode the completely identical amino acid sequence. However, based on our analysis, it is becoming clear that these three genes are finely used for different purposes at different times and in different situations.
While the existence (= expression) of hht1 gene is required in order to promote germination, hht1 expression level is rather decreased at the initial stage of germination as explained in section 4.3.4. Is there a meaning for hht1 expression level to get downregulated instead of getting upregulated at the beginning of germination? To answer this question, we performed gene modification, and created cells that do not decrease in hht1 expression level at the time of cellular awakening. These cells exhibited a remarkable delay in germination, which was a similar defect observed with the knock-out cells that do not express hht1 at all. These experimental results suggest that hht1 expression is downregulated during the initial stage of germination and upregulated afterwards at the middle to late stage, and that both decrease and increase in hht1 expression are indispensable for efficient germination. Then, why does the decrease in histone H3 expression connect to cellular awakening?
Generally, it is known that when histone octamers that coil to DNA decreases and chromatin structure loosens (chromatin becomes open), gene expression is promoted. On the other hand, when chromatin structure tightens (chromatin becomes closed), there is a tendency for gene expression to be suppressed. By analogy, it can be imagined that by the decrease in histone H3 level at the initial stage of germination, chromatin structure loosens and gene expression is upregulated at the entire genome range (Fig. 4-6). It is considered that by inducing a large-scale gene expression, cellular awakening from dormancy proceeds.
4.3.6 Future outlook
In order to validate whether the above inference led by the bioinformatics analysis (dry analysis) is accurate or not, we are currently planning an experiment to visualize the state of chromatins (wet analysis). Furthermore, we are considering to proceed with the validation through experiments (wet analysis) regarding the genes other than hht1 as well, in which the bioinformatics analysis revealed their expression changes during the initial stage of germination. Through these verifications, we hope to investigate what kind of regulation mechanisms cells undergo and wake up, and illustrate the overall picture of cell fate determination. As mentioned at the beginning, cellular awakening is a universal phenomenon of life that is not only seen in yeast, but also in animal and plant cells. For cellular dormancy and awakening, it appears crosstalk of various cellular phenomena, such as environmental adaptation, cellular life span, and fate determination, are happening closely. Indeed, we are convinced that the significance of the research will grow in the future.
Wake-up alarm: virtual time-lapse gene expression landscape illuminates mechanisms underlying dormancy breaking of germinating spores
Hayato Tsuyuzaki, Ryosuke Ujiie and Masamitsu Sato
Current Genetics (2021) 67(4):519-534. DOI:10.1007/s00294-021-01177-0
Time-lapse single-cell transcriptomics reveals modulation of histone H3 for dormancy breaking in fission yeast
Hayato Tsuyuzaki, Masahito Hosokawa, Koji Arikawa, Takuya Yoda, Naoyuki Okada, Haruko Takeyama and Masamitsu Sato
Nature Communications (2020) 11:1265. DOI:10.1038/s41467-020-15060-y
Mechanisms for Cellular Wake-up in Fission Yeast Revealed by Single-cell RNA-seq
Hayato Tsuyuzaki, Masahito Hosokawa, Haruko Takeyama and Masamitsu Sato
Bioscience and Industry (B&I) (2020) 78(6):507-509.
(※article only in Japanese)
