![]()
1.1 Mitosis and Meiosis
1.2 Cell cycle progression of mitosis and meiosis
1.3 Abnormal chromosome segregation in meiosis
1.4 The Importance of Microtubules in the Yeast Meiosis
1.5 Relationship between Meiotic abnormalities and Infertility in Mammals
1.1 Mitosis and Meiosis
There are two types of cell division. One of them, “mitosis”, is a type of cell division that has been repeated countless times in the formation of our bodies. It is a type of cell division in which one original cell (mother cell) produces two cells that are identical to itself (daughter cells).
In contrast, “meiosis” is an essential type of cell division process for gametogenesis. In this case, the purpose is not the production of cells that are identical to the original cell. Somatic cells in general are called diploid, for it contains two sets of chromosomes (genetic information) derived from both parents. On the other hand, gametes produced by meiosis only have one set of chromosomes, called a haploid. The fertilization of two haploid gametes (sperm and oocyte) creates a new zygote which is a diploid (1+1=2), leading to the birth of life. Hence, gametes must carry only a single set of chromosomes, making the production of haploid cells from a diploid cell, or in other words, ploidy reduction, as the greatest objective of meiosis. The purpose of meiosis is never about proliferation through formation of cells identical to their diploid mother cell.
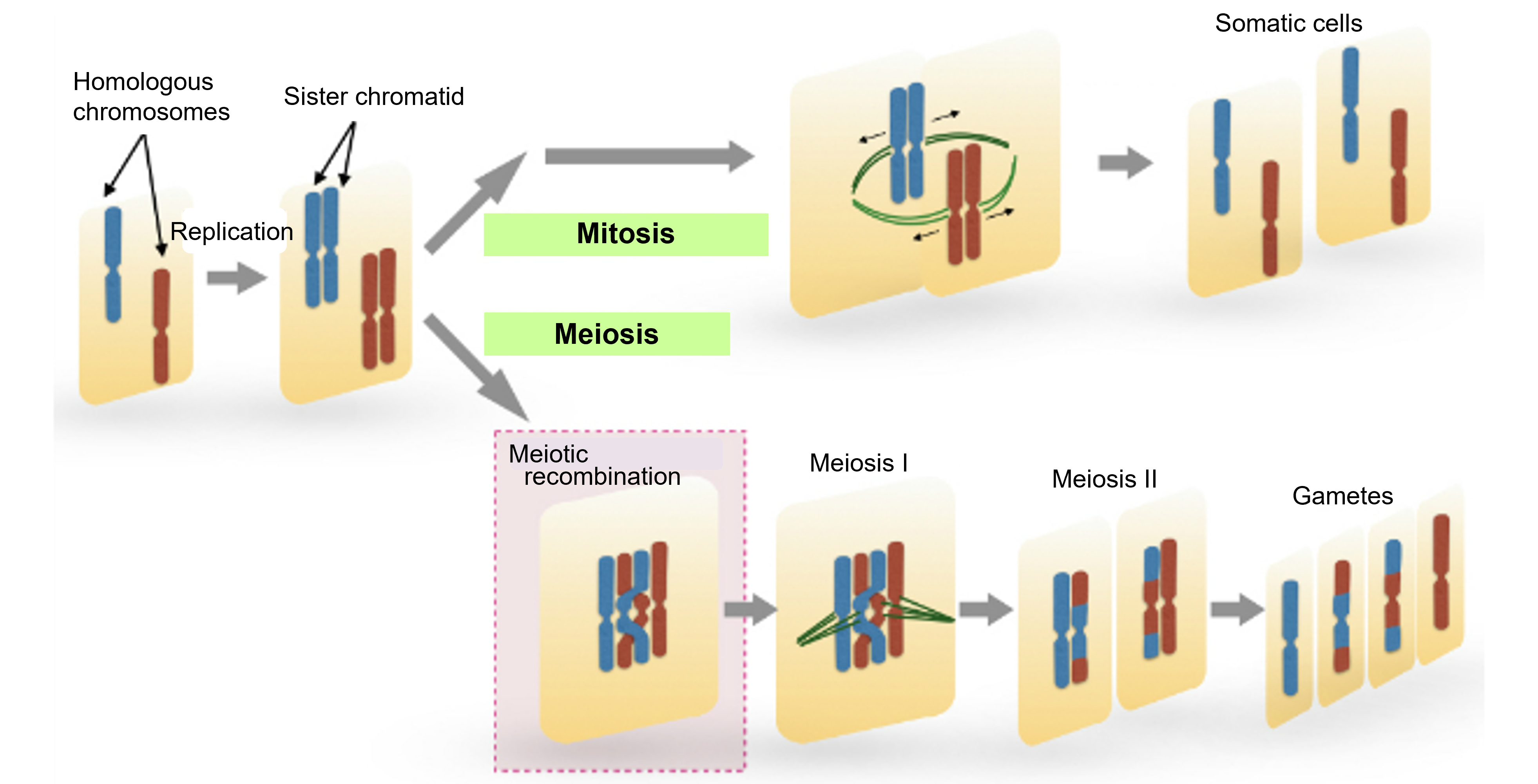
Figure 1-1 How the difference in multiplicity between mitosis and meiosis is created
Schematic representation of the difference between mitosis and meiosis. Homologous chromosomes (red and blue) in a single cell undergo DNA replication to achieve mitosis (top) or meiosis (bottom). In meiosis, homologous chromosomes pair up and undergo meiotic recombination. This is followed by two rounds of cell division, meiosis I and meiosis II, resulting in the production of gametes.
1.2 Cell cycle progression of mitosis and meiosis
These two types of cell division are widely conserved in eukaryotes, from yeast to humans. How are these two types of ordered cell division regulated so that they are carried out correctly? We are focusing particularly on the differences between the two types of division in our research.
(1) What are the differences between mitosis and meiosis?
(2) What genes are responsible for the differences?
(3) What is the purpose of the differences?
When we observe cells with these three questions in mind, we would recognize that even the very small cells only visible under a microscope have a tightly regulated molecular system that guarantees they divide at the right time and in the right way.
So far, we have studied the differences between mitosis and meiosis in fission yeast, which is a model organism used for studies on meiotic mechanisms (many of these research were conducted by Prof. Sato during his enrollment at the Department of Biophysics, Graduate School of Science, University of Tokyo).
For the case of fission yeast, the cell fate depends on their culture environment. Under nutrient-rich conditions, the cells proliferate by repeating mitosis (Figs.1-2 and 1-3). On the other hand, under nutrient-poor conditions, the cell cycle is arrested in G1 phase, and the cells are destined to enter meiosis. After G1 arrest, the diploid cell undergoes meiosis I, followed by meiosis II through DNA replication and meiotic recombination. After the two divisions, the chromosome ploidy becomes "haploid" and the cell produces spores (gametophytes).
Yeast can proliferate through mitosis at haploid state, but usually, this does not immediately initiate meiosis under nutrient starvation. For the case of haploid cells, they first mate to form diploid cells. In general, cells must be diploid in order to undergo meiosis, which is not surprising given that the original purpose of meiosis is to reduce chromosome ploidy (i.e., to make a diploid cell into a haploid cell). The apparent differences between the cell cycles of mitosis and meiosis are:
(1) Meiosis involves "meiotic recombination" (Fig.1-1)
(2) Meiosis involves two nuclear divisions, meiosis I and meiosis II, without DNA synthesis occurring in between them (Figs.1-2 and 1-3).
Through meiotic recombination in (1), homologous chromosomes are physically paired, and part of the paired chromosomes are exchanged (gene conversion). (2) refers to the difference of S phase between mitosis and meiosis. There is always an S phase (DNA synthesis phase) in between mitosis, while in meiosis, there is no S phase between meiosis I and meiosis II.
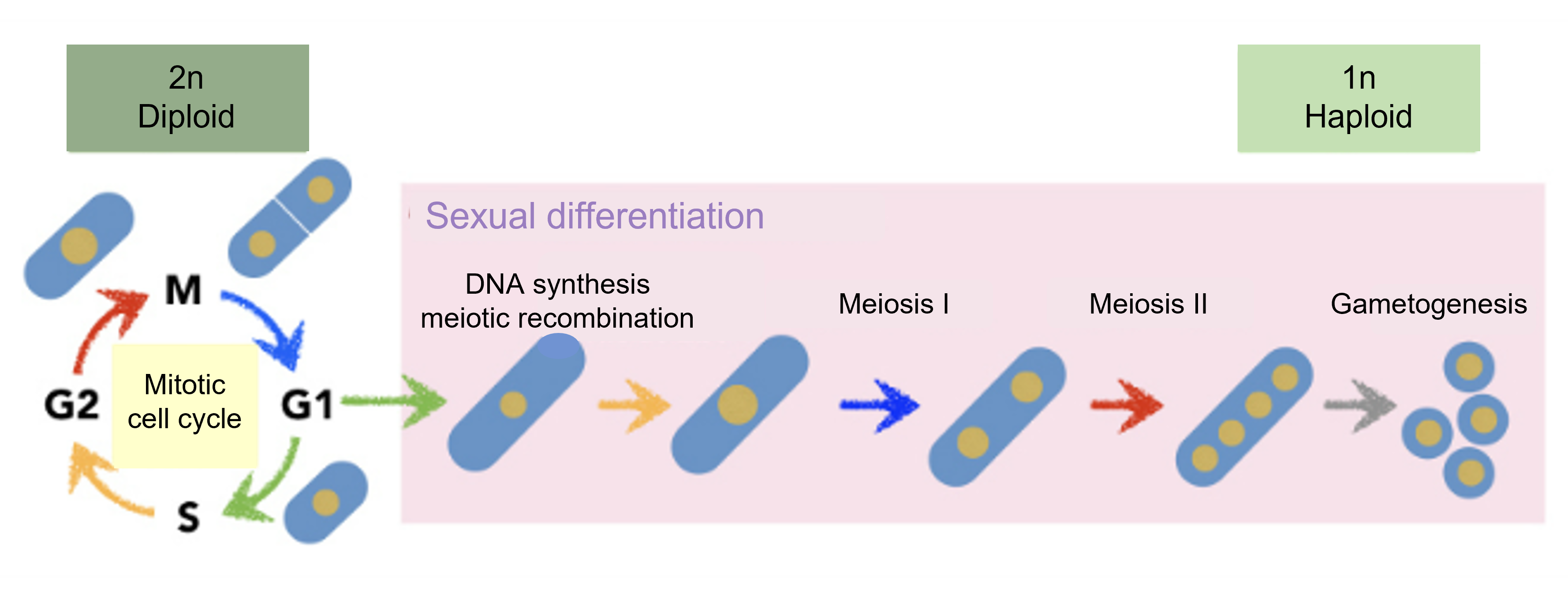
Figure 1-2 Cell cycle progression of general mitosis and meiosis
Diploid cells are arrested in G1 and enter sexual differentiation. After meiosis I, the cells undergo meiosis II in the absence of DNA synthesis, followed by gametogenesis.
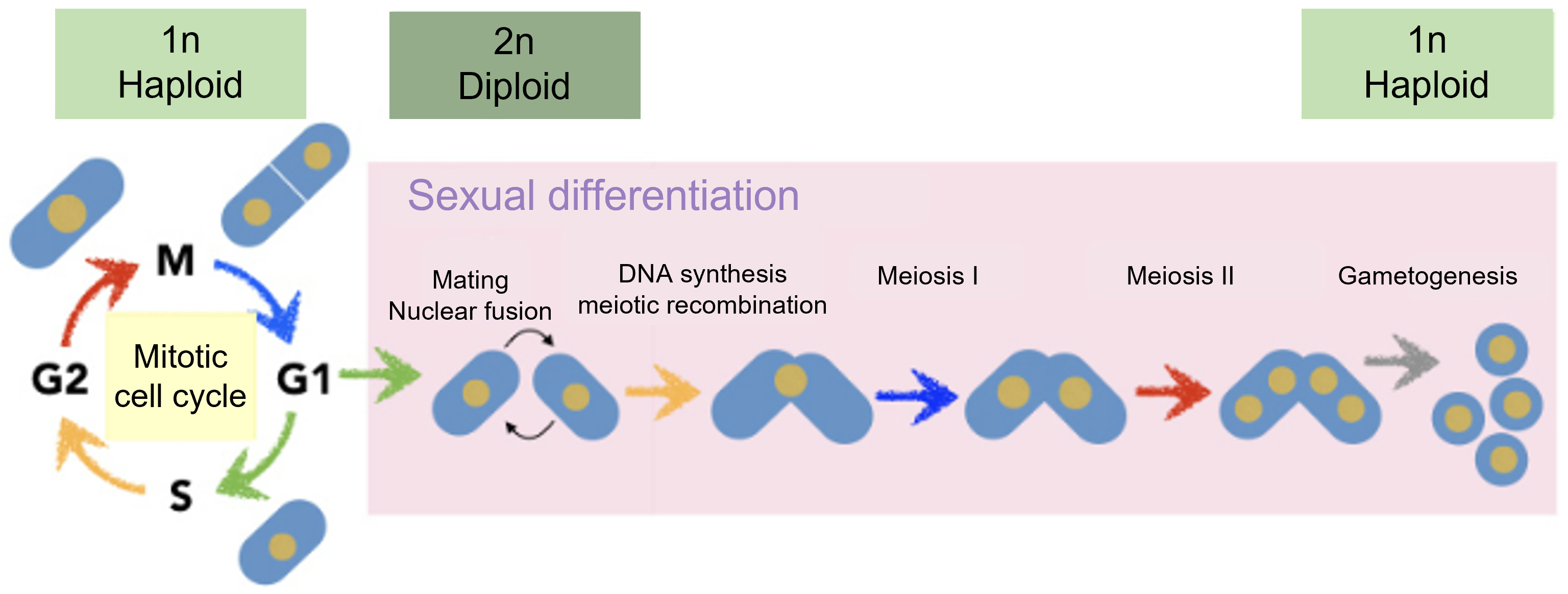
Figure 1-3 Cell cycle progression of mitosis and meiosis in fission yeast
Fission yeast can grow as haploid cells, and fuse together to form diploid cells under nutrient starvation. Diploid cells then undergo meiosis (meiosis I and meiosis II ) to form spores.
We also looked for phenomena that are only observed in meiosis, and elucidated their molecular mechanisms. For example:
(A) In meiosis, there is a massive reorganization of factors that make up the SPB (spindle poles), which correspond to the centrosomes.
(B) Meiosis II does not require pole-to-pole microtubules in the spindle microtubules (in meiosis II, chromosome segregation is possible with a mechanism that is less dependent on microtubules).
(C) Why does meiosis III not occur? We have identified that certain mutants undergo an abnormal meiosis III, and have been analyzing the significance and mechanism of this phenomenon.
Related Publication:
About (A):
Spindle pole body components are reorganized during fission yeast meiosis
Midori Ohta, Masamitsu Sato and Masayuki Yamamoto
Molecular Biology of the Cell (2012) 23(10):1799-811. DOI:10.1091/mbc.E11-11-0951
About (B):
Interpolar microtubules are dispensable in fission yeast meiosis II
Takashi Akera, Masamitsu Sato and Masayuki Yamamoto
Nature Communications (2012) 3:695. DOI:10.1038/ncomms1725
About (C):
Cuf2 boosts the transcription of APC/C activator Fzr1 to terminate the meiotic division cycle
Yuki Aoi, Kunio Arai, Masaya Miyamoto, Yuji Katsuta, Akira Yamashita, Masamitsu Sato and Masayuki Yamamoto
EMBO Reports (2013) 14(6):553-560. DOI:10.1038/embor.2013.52
1.3 Abnormal chromosome segregation in meiosis
Abnormal chromosome segregation in mitosis has been implicated in carcinogenesis. In contrast, abnormal chromosome segregation in meiosis is considered to be a cause of miscarriage, infertility, and trisomy-type congenital chromosomal disorders such as Down syndrome. Human Down syndrome patients contain an extra third copy of chromosome 21, called trisomy, instead of two. There are several possible reasons for trisomy. For example, during the process of meiosis producing sperm or oocytes, abnormal chromosome segregation may lead to the formation of a gamete containing two sets of chromosome 21. Fertilization of this gamete (1+2=3) may then result in trisomy.
1.4 The Importance of Microtubules in the Yeast Meiosis
What causes chromosome segregation errors during meiosis? In some cases, the cause is the same between mitosis and meiosis, but there should also be causes that are specific to meiosis. We hypothesize that the system of meiosis itself has some risks of causing chromosome segregation errors. In order to investigate the identity of these risks, we use fission yeast (an excellent model organism for research on meiosis) in our research.
The results of our research using fission yeast have revealed microtubules play a special role in meiosis to reduce the risk of chromosome segregation errors.
Upon initiating this research, we first considered what the risk of chromosome segregation errors might be, and that the risk may occur during meiotic recombination, a phenomenon unique to meiosis.
We considered the key question was: "where are the chromosomes located in the nucleus?” Normally, the kinetochores of chromosomes are tethered at the spindle pole body (called SPB or centrosome) during interphase (Fig.1-4, upper panel). This configuration of chromosomes is called the Rabl-like orientation, and it is considered as an excellent preparation for a faithful chromosome segregation. When cells enter mitosis, spindles microtubules emanate from the spindle poles, which can capture kinetochores immediately so that all chromosomes are equally segregated to daughter cells.
On the other hand, the chromosomes are located at a completely different position during meiosis. As a preliminary step in meiosis, chromosomes undergo "meiotic recombination". At this point, telomeres at the ends of chromosomes are clustered around SPBs, whereas kinetochores are positioned far away from SPBs (Fig.1-4, lower panel). This arrangement of chromosomes is named bouquet orientation, which is considered as an essential chromosomal state for meiotic recombination. However, considering the chromosome segregation that comes afterwards, we reasoned that it would be difficult for spindle microtubules to capture kinetochores if they are positioned far away from the SPBs, causing a risk of chromosome segregation errors.
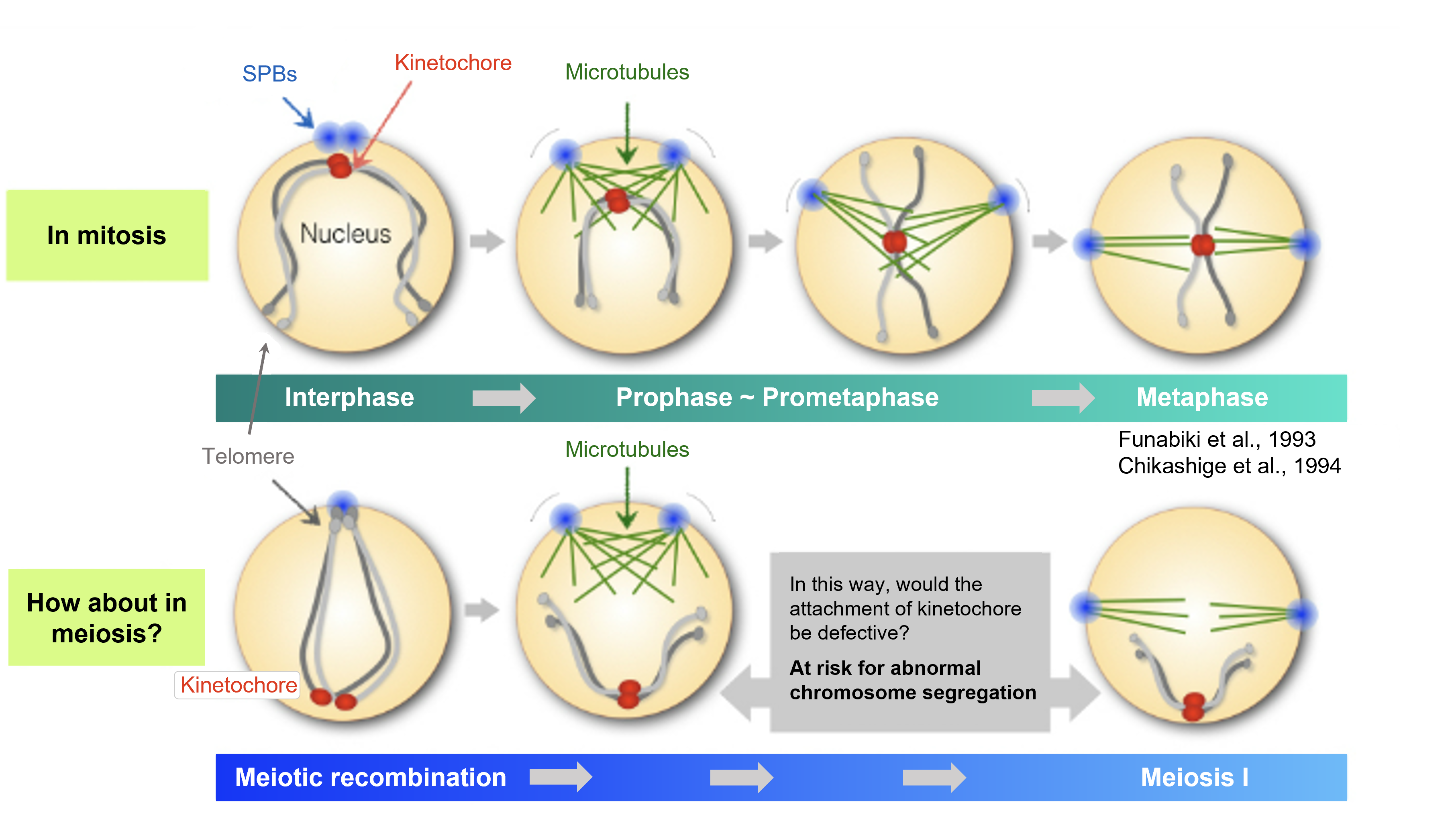
Figure 1-4 In meiosis, the chromosome configuration in the nucleus different from that in mitosis may impose a risk of meiotic failure
In mitosis, kinetochores of chromosomes are already tethered at the SPBs during interphase. In contrast, the kinetochore is located far away from the SPBs in meiosis, which may impose a high-risk chromosome segregation if the state is maintained.
Then how do cells reduce this risk? To identify the underlying molecular mechanisms, we established a live-cell imaging system which uses a fluorescence microscope (Sato, Toya and Toda, Methods in Molecular Biology., 2010). By using this system, we visualized various organisms in living cells with fluorescent proteins such as GFP (green), RFP (red), and CFP (blue), and focused on cells at the stage of transition from meiotic recombination to meiosis (meiosis I). As a result, we found that at the end of the recombination phase, long radial microtubules, which have a different structure from spindle microtubules, are emanated from SPBs and capture distant kinetochores so that chromosomes are retrieved towards SPBs (Fig.1-5 ①).
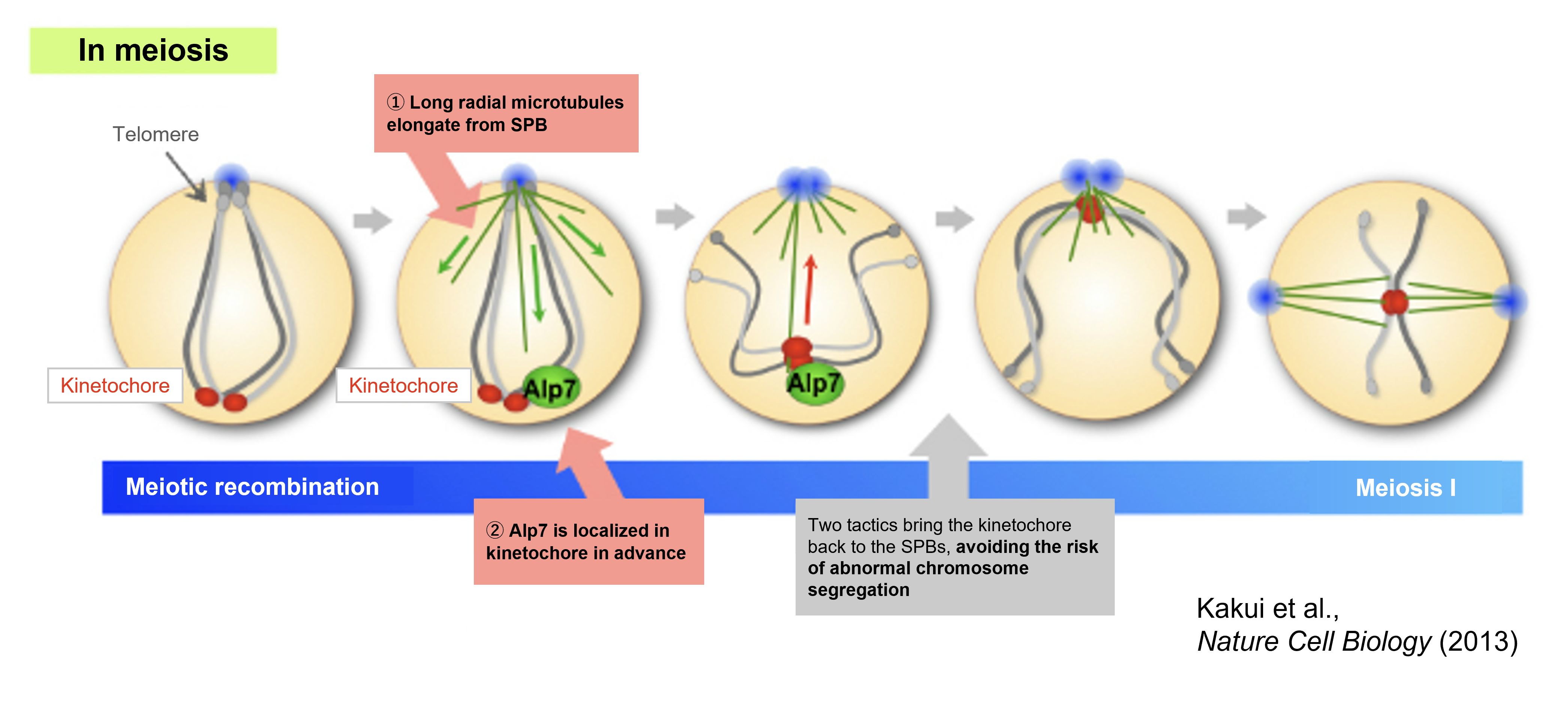
Figure 1-5 Problems in meiotic chromosome configuration is overcome by microtubules and their binding protein Alp7
Cells are equipped with two mechanisms to resolve these risks. ① Emanate radial microtubule array from the SPBs that capture distant kinetochores and retrieve them towards SPBs. ② Localize the microtubule-associated protein Alp7 to unattached kinetochores in advance to facilitate kinetochore-microtubule attachment
In other words, kinetochores distant from the SPBs will be a threat of chromosome segregation errors. Hence, it is considered that by emanating microtubules that retrieve distant kinetochores towards SPBs and reconstituting chromosome configuration to a Rabl-like orientation, efficient chromosome segregation is achieved.
Then, is this "retrieval" of chromosomes by radial microtubules really essential? We observed cells at the stage of meiosis using a mutant with extremely destabilized microtubules, and discovered that if microtubules are not formed, kinetochores are left at a distant position, resulting in chromosome segregation errors in the subsequent meiosis I. Therefore, it can be suggested that radial microtubules have an essential role in meiosis.
This was not the only the “tactic” for cells to safely undergo meiosis. In order for radial microtubules to successfully capture and retrieve the kinetochores, the microtubule-associated protein Alp7 (a factor equivalent to the human TACC3 protein) was found to be pre-localized to kinetochores, waiting for the arrival of microtubules (Fig.1-5 ②). Since Alp7 (TACC3) is a microtubule-binding protein, its localization to kinetochores that are distant from the microtubules is unusual, and represents a "tactic" unique to meiosis.
During the mitotic prophase, Polo-like kinase (Plo1), a mitotic phosphatase, also localizes to the kinetochores. It was found that this Plo1 phosphorylates Alp7, thereby pre-positioning Alp7 at kinetochores.
At this point as well, the issue is whether there really is a meaning for Alp7 to localize to kinetochores. We created Alp7 mutants, that are unaffected by phosphorylation by Plo1. When microtubules elongate in this mutant, the attachment of kinetochores to microtubules was possible for a moment, but the kinetochores soon slipped off the microtubules. Thus, it was demonstrated that the loading of Alp7 onto unattached kinetochores in advance promotes kinetochore-microtubule association.
With these two "tactics," it can be suggested that faithful chromosome segregation can be accomplished in meiosis, which is a process with high risks of chromosome segregation errors. The results of this research were published in Nature Cell Biology, and were introduced in several newspapers and websites (Waseda University PR website).
Related Publication:
Tell the Difference Between Mitosis and Meiosis: Interplay Between Chromosomes, Cytoskeleton, and Cell Cycle Regulation
Masamitsu Sato, Yasutaka Kakui and Mika Toya
Frontiers in Cell and Developmental Biology (2021) 9(10):660322. DOI:10.3389/fcell.2021.660322
Differentiating the roles of microtubule-associated proteins at meiotic kinetochores during chromosome segregation
Yasutaka Kakui and Masamitsu Sato
Chromosoma (2016) 125(2):309-20. DOI:10.1007/s00412-015-0541-x
Microtubules and Alp7-Alp14 (TACC-TOG) reposition chromosomes before meiotic segregation
Yasutaka Kakui, Masamitsu Sato, Naoyuki Okada, Takashi Toda and Masayuki Yamamoto
Nature Cell Biology (2013) 15(7):786-796. DOI:10.1038/ncb2782
Spindle pole body components are reorganized during fission yeast meiosis
Midori Ohta, Masamitsu Sato and Masayuki Yamamoto
Molecular Biology of the Cell (2012) 23(10):1799-811. DOI:10.1091/mbc.E11-11-0951
Visualization of fluorescence-tagged proteins in fission yeast and the analysis of mitotic spindle dynamics using GFP-tubulin under the native promoter
Masamitsu Sato, Mika Toya and Takashi Toda.
Methods in Molecular Biology - Mitosis (2009) 545:185-203. DOI:10.1007/978-1-60327-993-2_11
Nowadays with the aging trend of pregnancy and childbirth, the deterioration of oocyte quality with age has become a greater deal of attention. In mammalian oocyte development, meiosis is arrested at prophase I over a prolonged period of time, from fetal stage to puberty. Afterwards, meiosis resumes, followed by ovulation and fertilization. The prolonged arrest at prophase Ⅰ leads to a decline in cell quality, which is specific to oocytes and not sperm. However, whether it be spermatogenesis or oogenesis, the number of chromosomes in gametes will be abnormal if chromosome segregation errors occur. From this, both sperm and oocytes can serve as the cause of infertility.
In any case, it appears human oogenesis involves a different kind of risk from yeast meiosis. Particularly, we aim to identify what kind of problems occur in aged oocytes, and to contribute to infertility treatment in the future. Currently, we collaborate with fertility clinics to understand the current situation of medical and fertility treatment. We established the "Network for Reproductive Medicine and Science" in collaboration with Dr. Junya Ito of Azabu University, School of Veterinary Medicine, who specializes in developmental engineering. Together, we study what the risk of abnormal chromosome segregation in mammalian meiosis is.
