![]()
2.1 From cells to tissues
2.2 How microtubules are organized specifically in epithelial cells
2.3 How cystic kidneys are formed (Function of epithelial microtubules in the kidney)
As described previously, our body consists of numerous cells. Cells are proliferated through rounds of cell divisions and differentiated to play specific roles individually. Through these processes, cells are assembled into tissues to function together as a unit. Tissues are classified into four types: epithelial, muscular, neural, and connective tissues. Epithelial tissues include epidermis which covers the outer surface of organs and the lumen which covers the inner surface (for instance, the inner surface in intestine means the side where digested foods contact).
Functions and regulation of microtubules have been widely studied using single cells such as yeast and cultured mammalian cells. For our previous studies using yeast, please refer to Project 1 and Project 3.
If cultured mammalian cells have been established from tissues, each cell is expected to possess the original characteristics of the organ (e.g., derived from fibroblasts, epithelial cells, neural cells, blood cells). This is a reason why cultured cells from distinct organs tend to display different organization of microtubules. This suggests that microtubule assembly is regulated in a cell type-specific manner.
It is important to consider that cells in tissues may communicate each other, as they assemble a mega-complex unlike single cells in a culture. Cultured cells, even though they are originated from tissues, may behave differently depending on their environment: on a culture dish in a laboratory, or in the native tissue in a living organism. Cultured epithelial cells are flat on a laboratory dish, whereas those in the original tissue are rather three-dimensional: they have a height and are tightly connected each other. This is a reason why studies using cultured cells in a laboratory dish may not elucidate all the aspects of life science, including regulatory systems of our bodies and causes of diseases.
It is still unclear how microtubule is organized in living organisms and how the organized microtubules contribute to functions of tissues. In this study, we aim to elucidate the details how microtubules are organized in cells from various mammalian tissues, with mouse as a model organism. By integrating knowledge therefrom, we hope to clarify roles of microtubule organization in individuals.
2.2 How microtubules are organized specifically in epithelial cells
Recent studies including our group clarified how microtubules play a pivotal role in the assembly of epithelial tissues. We are now investigating the relationship between microtubules and diseases in tissues, based on the results of our previous studies.
Epithelial cells are one of the main components of internal organs, such as stomach, intestine, lung and kidney. Those organs physiologically play vital roles in secretion of enzymes and hormones, absorption of nutrients, and barrier to the outside. An essential structural key to fulfill this role is the apical-basal polarity of epithelial cells. The apical surface is faced with the outside air and body fluids, and the basal surface adheres to connective tissue (Fig. 2-1).
In interphase (the period of non-dividing phase), microtubules of typical eukaryotic cells are assembled in a mesh-like pattern in the cytoplasm, whereas those of intestinal epithelial cells are particularly aligned in a polarized manner along the apical-basal axis. This can be compared to a curtain, with the one end (the minus end) of microtubules anchored at the apical surface and the other end (the plus end) exposed toward the basal side (Fig. 2-1).
The polarized array of microtubules in epithelial cells was reported in the 1980s, but it remained unclear what types of factors or mechanisms are responsible for the array, and what the significance of the array is.
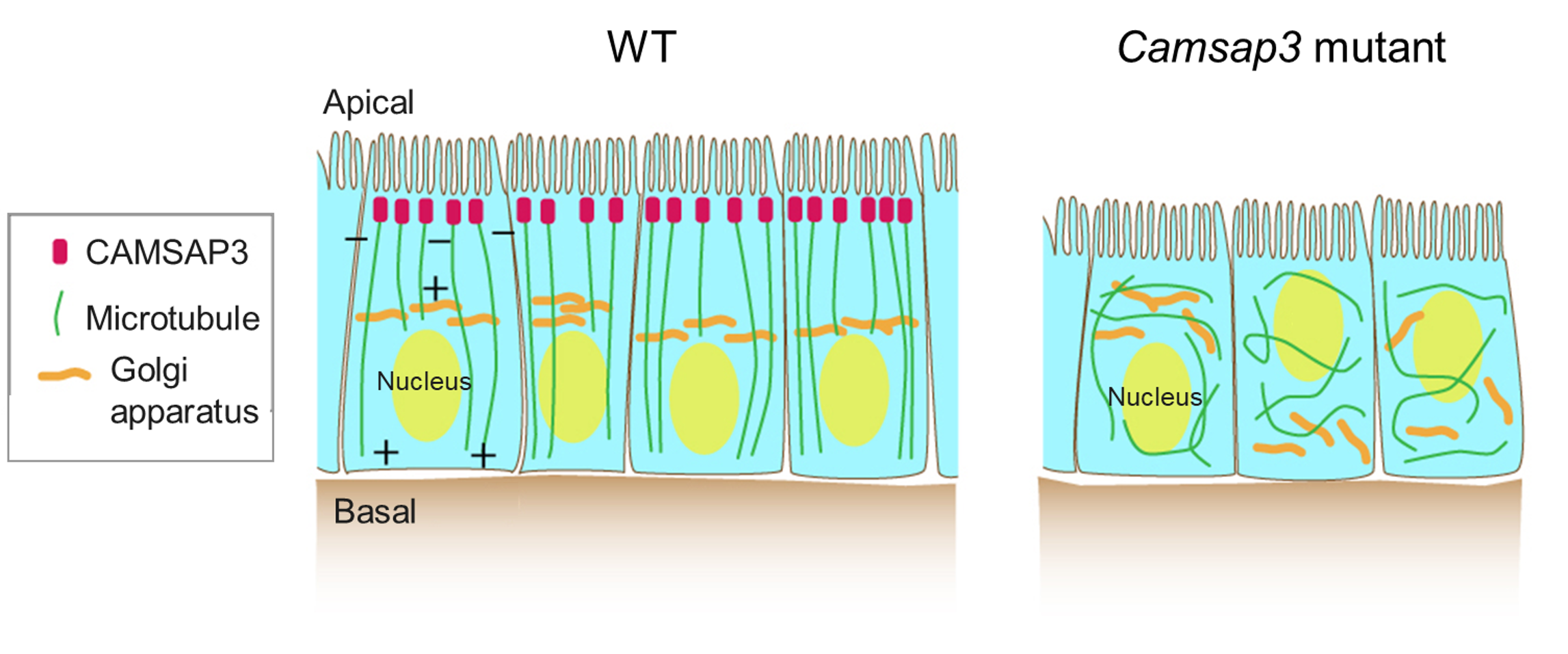
Figure 2-1 CAMSAP3 is responsible for the characteristic microtubule array in intestinal epithelial cells
Cross sectional views of intestinal epithelial cells in WT (wild- type) and Camsap3 mutant mice are depicted. In WT, microtubules are aligned in a polarized manner along the apical-basal axis. This is because the CAMSAP3 protein tethers the minus end of microtubules at the apical surface. In the Casmap3 mutant mice, the orientation of each microtubule is randomized in the cytoplasm and, accordingly, the intracellular arrangement of cell organelles is severely affected.
In 2016, the associate professor Dr. Toya in our laboratory reported that the microtubule minus end binding protein CAMSAP3 is responsible for the polarized array of microtubules in intestinal epithelial cells. Experiments with Camsap3 mutant mice and Caco-2 cells (an epithelial cell line from human colon cancer) revealed two facts below. (News and Announcements from the CDB)
1. CAMSAP3 localizes to the apical surface and tethers an end of microtubules in intestinal epithelial cells.
2. In the absence of the CAMSAP3 function, microtubules are no longer tethered at the apical surface and internalized into the cytoplasm. The orientation of each microtubule is randomized in the cytoplasm and, accordingly, the intracellular arrangement of cell organelles such as the nucleus and the Golgi apparatus is severely affected.
CAMSAP3 thus creates the polarity of microtubules by tethering them at the apical surface, thereby defining the position of various organelles in cells. A manuscript describing these results was published in the scientific journal Proc. Natl. Acad. Sci. USA and was then featured on the RIKEN NEWS, the American Society for Cell Biology (ASCB) website and in F1000prime (Faculty Opinions), an international peer-reviewed publication evaluation system.
In the small intestine, new cells are constantly born at crypts, located at the very bottom of the parenchyma even in adults. Older cells are shed from the tips of the parenchyma, and thus new and old cells are metabolically circularized (Fig. 2-2). As new cells are generated through division of stem cells in crypts, spindle microtubules for the division are expected to be reorganized into the polarized array in the newborn cells after division. This could be an interesting issue, and we hope that combinations of many experimental systems with intestinal tissues, stem cells and three-dimensional cultures of epithelial cell lines, will clarify the molecular mechanisms of microtubule regulation in epithelial cells, which are expected to understand the physiological role of the characteristic organization of microtubules. These studies are being conducted in collaboration with Dr. Masatoshi Takeichi at RIKEN Centre for Biosystems Dynamics Research (RIKEN BDR).
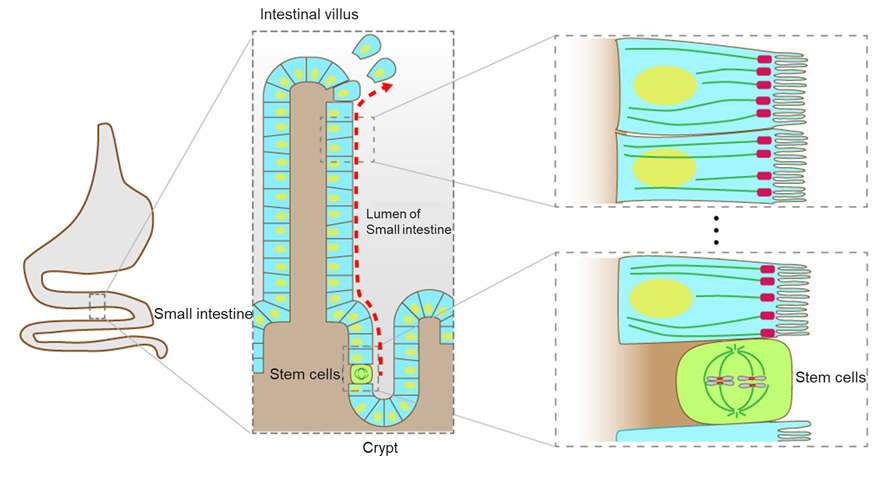
Figure 2-2 Newborn cells generated through division of stem cells are moved toward villus to be metabolically circularized in intestinal epithelial tissue
The inner surface of the intestine contains numerous crypts and villi. Intestinal epithelial cells, generated through division of stem cells in crypts, are extruded from the crypts into the villi and, finally, are shed from the tips of the parenchyma. In stem cells, microtubules are organized to form spindle to segregate chromosomes and complete cell division. Epithelial cells generated through division of stem cells are expected to have the polarized array of microtubules. In this figure, cells except absorptive epithelial cells and stem cells are omitted for simplicity.
Interestingly, most cancers (carcinoma) are known to arise in epithelial tissues. Considering that the microtubule-associated protein APC (adenomatous polyposis coli) has been identified as a protein involved in the development of colorectal cancer, and that microtubule polymerization inhibitors are used in cancer therapy, researches on microtubules in the epithelium is getting even more important toward the future.
2.3 How cystic kidneys are formed (Function of epithelial microtubules in the kidney)
Kidney is an important organ that removes waste products from the blood and regulates homeostasis of water and salt in the body. Cystic kidney is a common lesion that almost half of people over the age of 50 have. The degrees of phenotypic traits are variable depending on individuals regarding the number of cysts and whether the cysts are seen in one or both kidneys. Although most cystic kidneys are benign and show no symptoms, some hereditary cystic kidneys and genes responsible for the traits have been reported. Researches have been conducted to elucidate their molecular mechanisms, but much is still unknown about how cysts are formed.
Through the analyses of functions of the CAMSAP3 protein in small intestine, we also found that both kidneys of elderly Camsap3 mutant mice are whitened and enlarged (Mitsuhata et al., Sci. Rep., 2021, also see: Department of Biomedical Sciences, School of Advanced Science and Engineering, Waseda University, Waseda University Research Topics). In young mutant mice, the appearance of the kidneys is similar to that of WT (wild type) kidneys, but when internal tissue sections are examined, numerous cysts are found in the cortical area. We emphasize the following discoveries are particularly of significance: the CAMSAP3 gene is responsible for cystic kidneys and that epithelial microtubules regulated by CAMSAP3 maintain the structure of kidneys.
Kidney is made of many fundamental structural units called nephrons. A nephron is composed of a renal corpuscle and connected tubules. The renal corpuscle is further divided into the glomerulus and its supporting Bowman's sac. The tubules are classified into the proximal tubule of the glomerular side, then the Henle loop, the distal tubule and the collecting duct, leading to the bladder.
Examination of kidneys from Camsap3 mutant mice revealed that proximal tubules were specifically dilated in the nephron (Fig. 2-3). It is known that approximately 70 % of the filtered urine is reabsorbed in the proximal tubules.
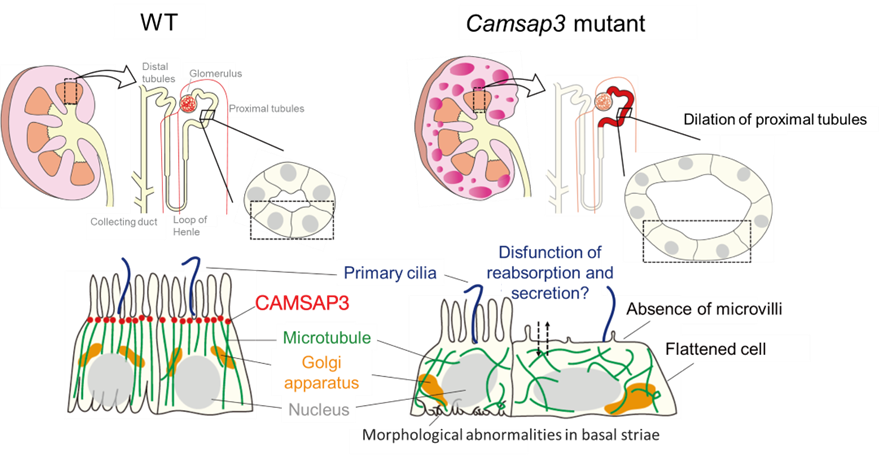
Figure 2-3 The structure of cystic kidney in Camsap3 mutant mouse
In Camsap3 mutant mice, proximal tubules are dilated and then cysts are developed. In dilated tubular epithelial cells, the microtubule orientation is perturbed, the morphology of the basal membrane incarceration structures (basal striae) shows abnormalities, and microvilli are absent at the apical surface.
What is happening in the dilated proximal tubules in cystic kidneys of Camsap3 mutant mice? Observations of the cellular architecture revealed that the epithelial cells of the dilated proximal tubules reduced the cell height and expanded the cell width, indicating that the cells were elongated and flattened (Fig. 2-3).
Observations of CAMSAP3 protein localization and microtubule morphology showed that CAMSAP3 localized to the apical surface, tethering microtubules in WT tubular epithelial cells, as seen in intestinal epithelial cells. In contrast, in dilated tubular epithelial cells in Camsap3 mutant mice, the mutant protein did not localize to the apical surface and microtubule orientation was perturbed. In addition, the basal membrane incarceration structures characteristic in tubules (basal striae) showed abnormalities in directionality and depth, and microvilli were absent at the apical surface.
The observed abnormalities in the cellular architecture may also affect the physiological function of kidneys, and therefore biochemical assays of urine and blood were performed. The results showed that Camsap3 mutant mice tended to have lower levels of K+ and Mg2+ in the blood but higher levels in the urine. On the other hand, no abnormalities were found in values monitoring renal filtration function, and further analysis is necessary to determine the effect of epithelial microtubules on renal function.
How are cysts formed in kidneys of Camsap3 mutant mice? Specifically, how do proximal tubules with the Camsap3 mutation dilate with flattening of the cells? We hypothesized that the number of cells might be increased. However, cell proliferation was observed in the kidneys of Camsap3 mutant mice, regardless of whether the tubules are dilated or not. It still remains unclear how the tubules were dilated.
We then focus on YAP, a transcriptional activator (regulator of gene expression). YAP is also involved in cell proliferation and maintenance of cell morphology against mechanical forces. Immunostaining for YAP showed its localization in the cytoplasm and excluded from the nucleus in WT mice, whereas YAP translocated to the nucleus in dilated tubules in Camsap3 mutant mice, suggesting that YAP was activated (Fig. 2-4). YAP localization remained cytoplasmic in nondilated proximal tubules of mutant mice, suggesting that YAP activation is associated with tubular dilation in mutant mice. Further analysis suggests that tension was exerted on the apical surface of the flattened epithelial cells (accumulation of MLC2 in the apical region, a factor regulating myosin activity) and localization of PIEZO1, known as a factor of mechanosensory systems, was also changed. Based on these results, currently, we consider the following mechanostress-induced proximal tubular dilation as a mechanism of cystic kidney formation due to the Camsap3 mutation (Fig. 2-4). Our study found that a smaller number of microtubules in WT epithelial cells of the proximal tubule, compared to those of the distal tubule and the collecting duct. For this reason, it is possible that the proximal tubules are more susceptible to the Camsap3 mutation than other regions. Loss of CAMSAP3 functions led to the perturbation of epithelial microtubules and, accordingly, impaired maintenance of the cell shape. Consequently, the tubules are sensitive to internal forces and then dilated in a region-specific manner (Fig. 2-4). The time when cysts begin to be observed in the mutant mice coincides with the time when fetuses begin to produce the primary urine. It is therefore possible that the flow of the primary urine generates the pressure to surrounding epithelial cells of the tubules. In addition, the increased cell proliferation in Camsap3 mutant mice, irrespective of the tubular region, may also contribute to the expansion of the cystic area with age.
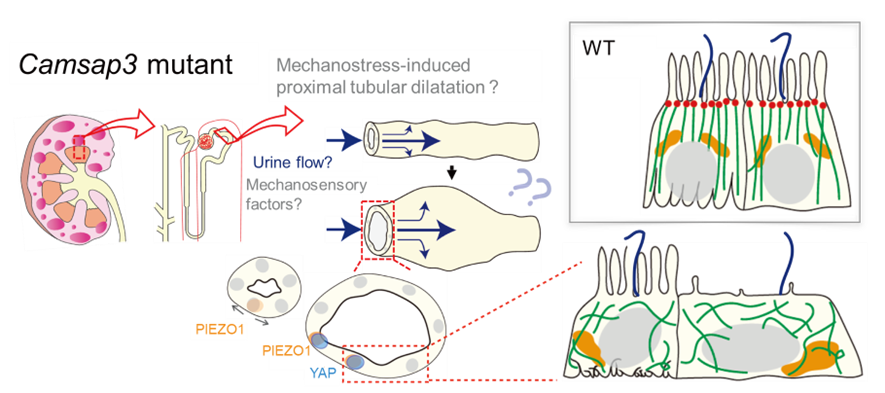
Figure 2-4 Model of how cysts are formed
Loss of Camsap3 functions perturbs the microtubule orientation in tubular epithelial cells. Consequently, the force to maintain the cell shape may become weak and therefore cells are unable to resist the pressure by the flow of the primary urine. This causes dilation of proximal tubules, which finally develops into cysts. The pressure of by the primary urine flow activates PIEZO1 and YAP, factors of mechanosensory systems. Because proximal tubules have fewer microtubules than the other tubules do, the effect of loss of Camsap3 might be prominent.
Currently, in collaboration with Takeyama Laboratory of the Department of Biomedical Sciences, we now research the mechanism of cystic kidney formation caused by the Camsap3 mutation from the viewpoint of gene expression. Microtubule perturbations in the Camsap3 mutation were also found not only in the kidney and small intestine but also in other organs. Further studies on the role of microtubule organization in epithelial cells of other organs are underway.
Related Publication:
Cyst formation in proximal renal tubules caused by dysfunction of the microtubule minus-end regulator CAMSAP3
Yuto Mitsuhata, Takaya Abe, Kazuyo Misaki, Yuna Nakajima, Keita Kiriya, Miwa Kawasaki, Hiroshi Kiyonari, Masatoshi Takeici, Mika Toya and Masamitsu Sato
Scientific Reports (2021) 11:5857. DOI:10.1038/s41598-021-85416-x
CAMSAP3 orients the apical-to-basal polarity of microtubule arrays in epithelial cells
Mika Toya, Saeko Kobayashi, Miwa Kawasaki, Go Shioi, Mari Kaneko, Takashi Ishiuchi, Kazuyo Misaki, Wenxiang Meng, and Masatoshi Takeichi
Proc. Natl. Acad. Sci.U.S.A. (2016) 113(2):332-337. DOI:10.1073/pnas.1520638113
Organization of non-centrosomal microtubules in epithelial cells
Mika Toya and Masatoshi Takeichi
Cell Struct and Function (2016) 41(2):127-135. DOI:10.1247/csf.16015
